Abstract
Background: The CHOICE Project was conducted in partnership between the US Centers for Disease Control and Prevention (CDC) and Hemophilia Federation of America, a non-profit, community-based organization, to survey persons with bleeding disorders (PWBD), male and female, in the US. Participants in the CHOICE Project included both PWBD receiving care at federally-funded hemophilia treatment centers (HTC) and those who do not receive care at HTCs (non-HTC PWBD). The ExPAND Project was derived to analyze a portion of the CHOICE data set related to males and females with a single diagnosis of hemophilia A or hemophilia B (PWH, HTC and non-HTC).
Objective: To retrospectively analyze treatment and healthcare utilization and perceptions among female PWH (FWH) in the CHOICE data set to better understand gaps in FWH healthcare and how those gaps can be addressed.
Methods: Demographic and clinical information was collected through CHOICE by survey in the US from 04/2013-07/2015 among adults (≥18 years) and caregivers of children with bleeding disorders. Participants were recruited to take a 20-minute survey in English or Spanish, online or on paper. Non-HTC PWBD were solicited specifically but others were not excluded from participation. Participants' status as non-HTC PWBD was determined using an algorithm based on responses to a specific survey question. Both females diagnosed with a bleeding disorder and undiagnosed females with bleeding disorder symptoms and/or children with a bleeding disorder could participate. Because participants were not required to respond to every survey questions, data for females with hemophilia A or B are combined in some instance to afford a sufficient n for analysis. Descriptive analysis of participants reporting hemophilia diagnosis was performed.
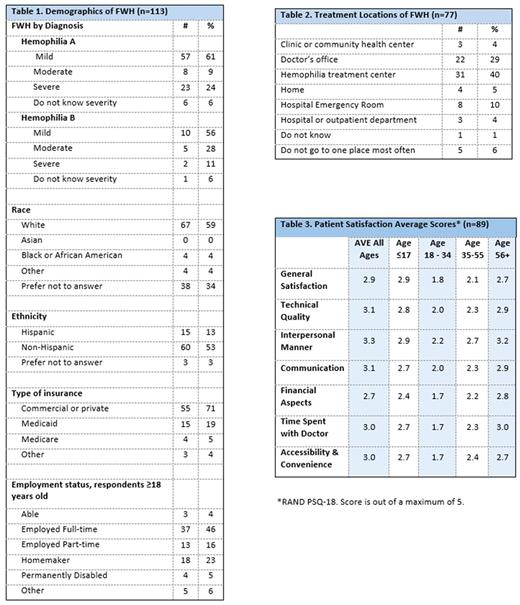
Results: Of the 719 eligible surveys, 576 PWH were identified as having a single diagnosis of hemophilia A or hemophilia B. Of the 576 PWH, FWH accounted for 20% (n=113); of FWH, 61% reported mild hemophilia A and 56% mild hemophilia B. FWH ethnicity was 13% Hispanic and race: 59% White, 4% Black/African American, 0% Asian, 4% other; 71% reported having commercial health insurance; 46% were employed full-time, 5% permanently disabled (Table 1). Of FWH, 77% use recombinant FVIII, 20% plasma-derived FVIII containing vWF, 11% hormonal contraceptives. Of FWH using factor products, 23% use it to prevent bleeds, 20% use it on a regular schedule, 57% infuse at home, and 77% self-infuse. Over 81% of FWH reported ever having had a bleed and in the prior 12 months 59% reported having a joint bleed and 46% a bleed in another location. Over 63% of FWH reported receiving care for their hemophilia at least once per year and 23% receive care only prior to a medical procedure. In the prior 12 months, due to their hemophilia 14% were admitted to a hospital and 28% visited an ER. For usual care, 40% visit an HTC, 29% a doctor's office, 10% ER (Table 2). For FWH, general satisfaction with care for their hemophilia was 2.9 out of 5 for all ages, with FWH ages 18-34 reporting 1.8 out of 5 (Table 3).
Conclusions: Preliminary data from CHOICE offers new insights in the treatment and healthcare utilization among FWH. As FWH in their reproductive years (18-34) had the lowest satisfaction score, there is further need to understand the disease/treatment paradigm for this group. Additional data for FWH of different race/ethnicity is also needed to properly assess these population. This sample does not necessarily represent all PWBD, as targeted outreach in some regions may have led to over-representation of some participant characteristics.
Morales Arias: Bioverativ: Employment. Cyhaniuk: HFA: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal